MSc in Medical Devices, Regulatory Affairs and Health Information Technologies
This comprehensive master's program combines engineering principles with biomedical applications. Students gain expertise in solving complex problems at the intersection of biology, medicine, and engineering to improve medical care delivery.
30
Credit Hours
100%
Online
3
Concentrations
12-24
Months


Industry-aligned curriculum designed with healthcare leaders
Flexible online learning with live virtual sessions
Hands-on projects with real-world applications
Career support and networking opportunities
Choose Your Concentration
Tailor your degree to match your career goals with one of our three specialized concentrations.
Medical Devices
Design, develop, and innovate cutting-edge medical equipment and devices that improve patient outcomes.
Regulatory Affairs/Quality Assurance
Master FDA regulations, international standards, and quality management systems for medical products.
Health Information Technologies
Lead digital transformation in healthcare through EHR systems, data analytics, and health informatics.
State-of-the-Art Facilities
Our partner labs provide students with access to industry-standard equipment for practical training.

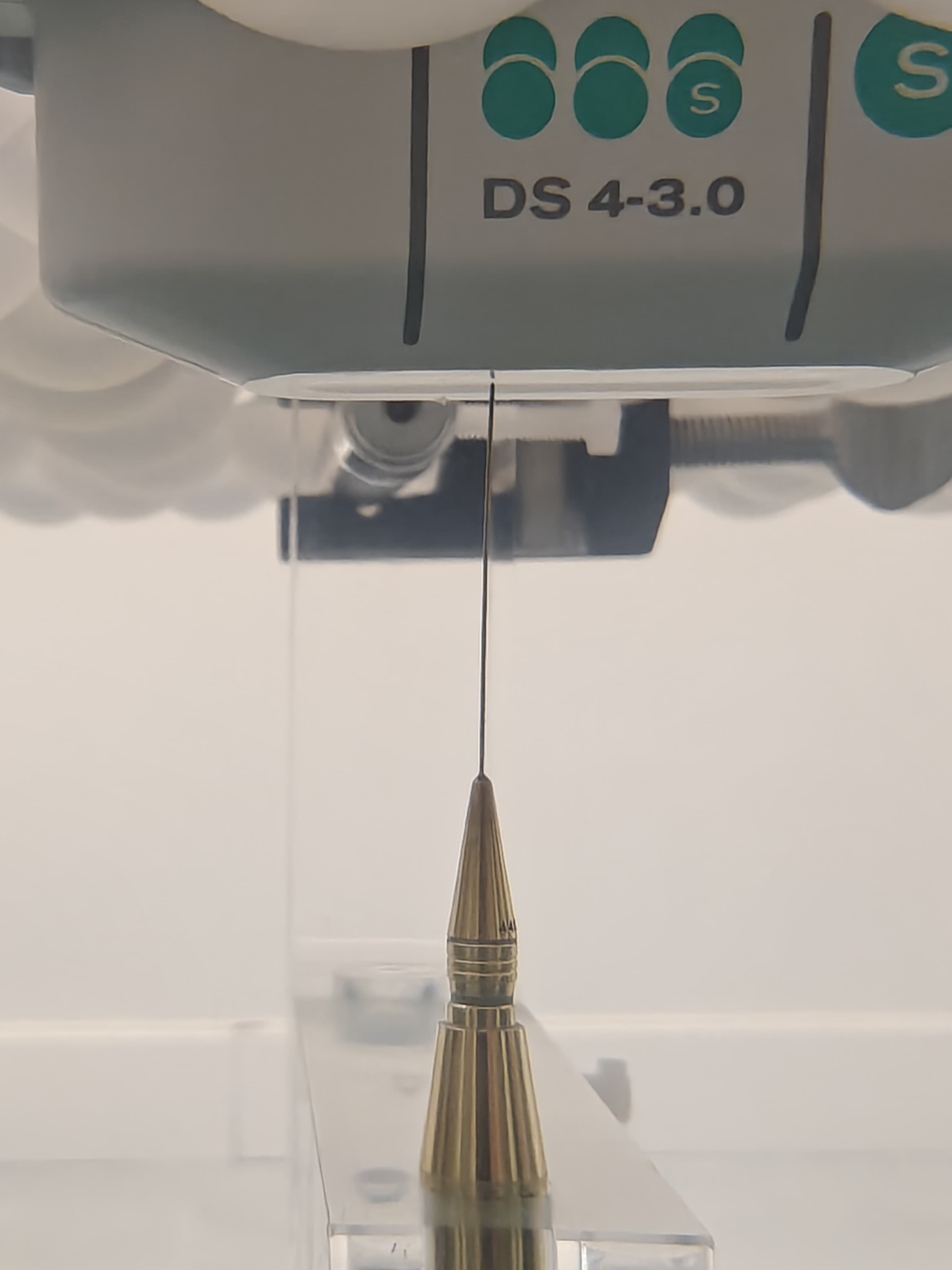
Medical Device Testing
Full-scale testing and compliance verification

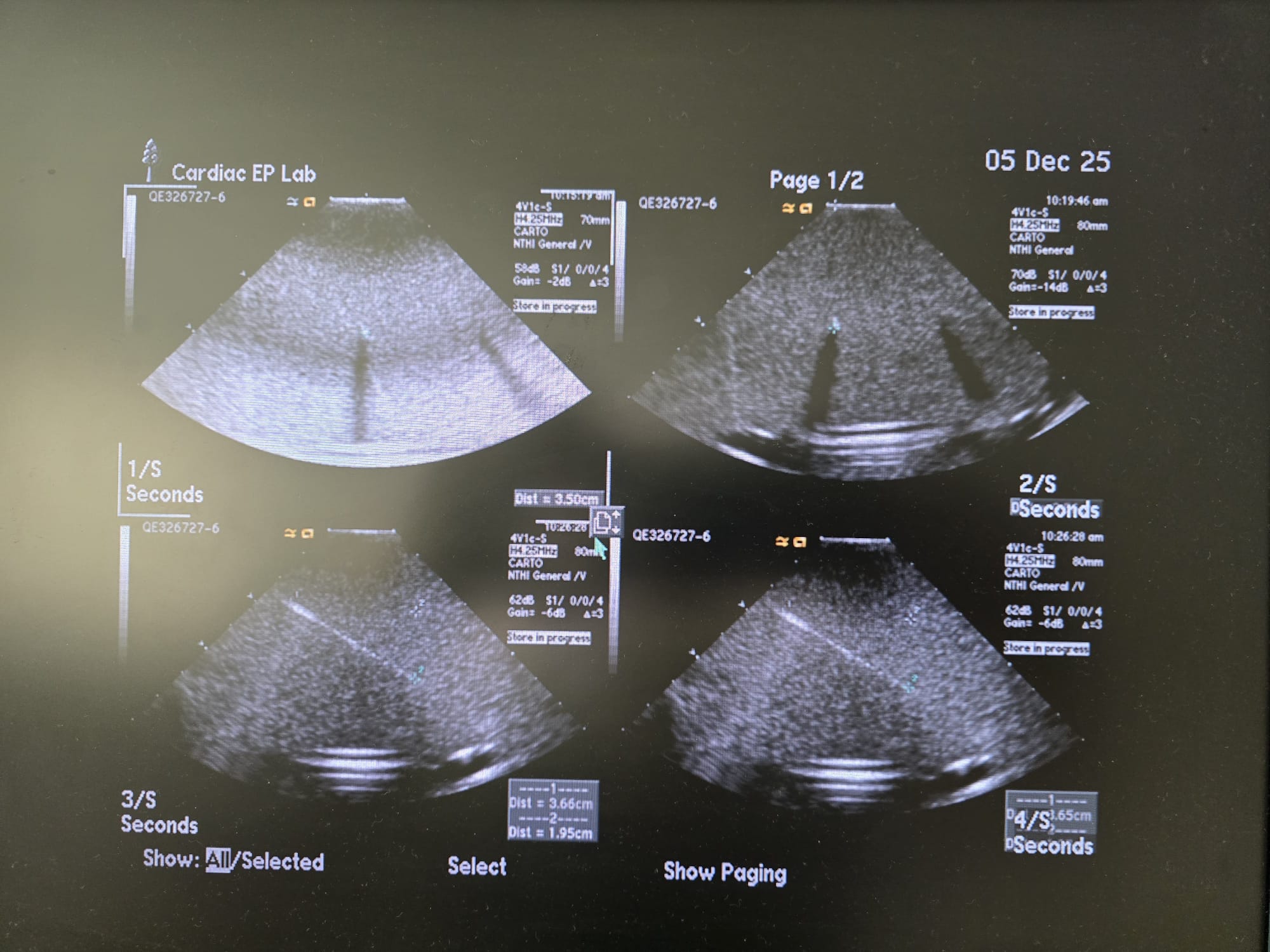



Hyperbaric Systems

Cardiac Imaging

3D Prototyping
Curriculum Overview
A comprehensive curriculum designed to prepare you for leadership in the medical device industry.
Semester 1
- Foundations of Biomedical Engineering
- Medical Device Regulations & Standards
- Healthcare Systems & Policy
Semester 2
- Medical Device Design & Development
- Quality Management Systems
- Health Information Systems
Semester 3
- Clinical Trials & Research Methods
- Risk Management in Healthcare
- Concentration Elective I
Semester 4
- Capstone Project
- Concentration Elective II
- Leadership in Healthcare Innovation
Learning Outcomes
Upon completion of this program, you will have mastered these essential competencies.
Engineering Excellence
Apply engineering, mathematics, and science principles to biological and medical challenges
Research & Development
Design and conduct biomedical engineering experiments with rigorous methodology
Device Innovation
Develop medical devices including prosthetics, pacemakers, imaging systems, and surgical instruments
Ethical Practice
Design biomedical components while considering ethical, social, and regulatory factors
Team Collaboration
Work effectively in multidisciplinary teams across healthcare and engineering
Communication Skills
Communicate technical information effectively to diverse audiences
Career Opportunities
Graduates pursue rewarding careers in the rapidly growing medical technology industry.
Biomedical Engineer
+6% growthDesign and develop medical equipment and devices
R&D Engineer
+8% growthLead innovation in medical technology products
Quality Assurance Manager
+7% growthEnsure medical devices meet regulatory standards
Clinical Engineer
+5% growthManage medical equipment in healthcare facilities
Regulatory Affairs Specialist
+9% growthNavigate FDA and international compliance
Health IT Manager
+12% growthLead digital health initiatives and systems
Frequently Asked Questions
What are the admission requirements?
Can I work while completing this program?
What technology or software will I need?
Are there networking opportunities?
Ready to Transform Your Career?
Join a community of innovators shaping the future of healthcare technology. Applications are now open for the next intake.
Explore Other Programs
Find the program that best fits your career goals